AHA 2025: Late-Breaking Data Reinforce the Prognostic Power of AI-Driven Heartflow Plaque Analysis as the Most Clinically Validated Framework for Coronary Risk Stratification
Multicenter outcomes FISH&CHIPS study of nearly 8,000 patients confirms total plaque volume as a powerful independent predictor of long-term cardiovascular events
NEW ORLEANS, Nov. 09, 2025 (GLOBE NEWSWIRE) -- Heartflow, Inc. (Heartflow) (Nasdaq: HTFL), the leader in AI technology for coronary artery disease (CAD), today announced late-breaking data from the FISH&CHIPS Study presented at the American Heart Association (AHA) Scientific Sessions 2025. The new data add to the robust and growing body of evidence supporting AI-powered Heartflow Plaque Analysis with Heartflow Plaque Staging* — the most clinically validated framework for actionable CAD care.
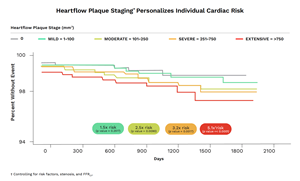
The retrospective analysis, which evaluated nearly 8,000 symptomatic patients from a cohort of the FISH&CHIPS Study, represents the largest validation to date of the Heartflow Plaque Staging framework based on total plaque volume (TPV) measurement.1
Key findings include:
- Patients in the highest TPV stage experienced more than a 5x greater risk of major cardiovascular events compared with patients in the lowest stage (hazard ratio 5.10, p-value < 0.0001).
- Higher plaque volume stages were independently associated with significantly increased rates of cardiovascular death and myocardial infarction over a median 3.3 years of follow-up.
- Associations remained significant after adjustment for coronary stenosis, FFRCT values and cardiovascular risk factors.
“This study provides strong validation of TPV-based staging measured with Heartflow Plaque Analysis as a predictor of future heart attacks or cardiovascular death,” said Timothy Fairbairn, Ph.D., principal investigator for the FISH&CHIPS study, Liverpool Heart and Chest Hospital, and Associate Professor at the University of Liverpool, UK. “The ability to accurately measure plaque will enable cardiologists to better predict which patients are most at risk above the traditional risk factors, and thus personalize treatment, in order to prevent heart attacks or death in the future.”
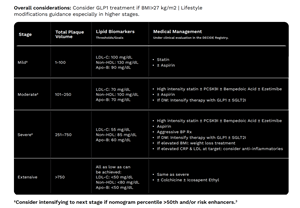
The findings build on results from the DECIDE Registry presented at the Society of Cardiovascular Computed Tomography (SCCT) 2025 Annual Scientific Meeting in July. DECIDE data showed that Heartflow Plaque Analysis with Plaque Staging led to changes in medical management for more than half of patients, resulting in an average reduction in LDL cholesterol of 18.7 mg/dL at 90 days. These results indicate management changes guided by Heartflow Plaque Staging result in an expected 15% decrease in risk of a cardiac event.2,3,4
“We are demonstrating how AI-powered Heartflow Plaque Analysis with Heartflow Plaque Staging can fundamentally change the way we manage CAD,” said Campbell Rogers, M.D., F.A.C.C., Chief Medical Officer at Heartflow. “These latest findings show that by embedding plaque insights directly into the diagnostic pathway, we can help physicians make more confident decisions to guide personalized and precise treatment for their patients.”
*Heartflow Plaque Analysis is an FDA-cleared device. Heartflow Plaque Staging is an investigational-only framework, and its safety and effectiveness have not been reviewed by the FDA.
About Heartflow’s Technology and Research
Heartflow’s technology is redefining precision cardiovascular care through clinically-proven AI and the world’s largest coronary imaging dataset. Heartflow has been adopted by more than 1,400 institutions globally and continues to strengthen its commercial presence to make this cutting-edge solution more widely available to an increasingly diverse patient population. Backed by ACC/AHA guidelines and supported by more than 600 peer-reviewed publications, Heartflow has redefined how clinicians manage care for nearly 500,000 patients worldwide. Key benefits include:
- Proprietary data pipeline: Built from more than 110 million annotated CTA images, Heartflow’s data foundation powers advanced AI models that deliver highly accurate, reproducible insights across diverse patient populations.
- Extensive clinical and real-world validation: Heartflow’s AI-driven solutions have been validated through clinical evidence in over 100 studies assessing over 365,000 patients. Proven in real-world practice with reproducibility and accuracy, Heartflow’s coronary CTA image acceptance rates exceed 96%.
- Seamless clinical integration via upgraded workflow: Heartflow delivers final quality-reviewed analyses instantly upon order, enabling clinicians to move from diagnosis to decision without delay.
- Quality system, global security and patient-data integrity compliance: Heartflow meets or exceeds leading international standards, including HITRUST, SOC 2 Type 2, GDPR, HIPAA, CCPA, ISO 13485, and ISO 27001.
About Heartflow, Inc.
Heartflow is transforming coronary artery disease from the world’s leading cause of death into a condition that can be detected early, diagnosed accurately, and managed for life. The Heartflow One platform uses AI to turn coronary CTA images into personalized 3D models of the heart, providing clinically meaningful, actionable insights into plaque location, volume, and composition and its effect on blood flow — all without invasive procedures. Discover how we’re shaping the future of cardiovascular care at heartflow.com.
Media Contact
Elliot Levy
elevy@heartflow.com
Investor Contact
Nick Laudico
nlaudico@heartflow.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/4702aa38-c48c-4291-91aa-937325c8206f
https://www.globenewswire.com/NewsRoom/AttachmentNg/a2a0e458-a265-4790-90b4-33f7403b0b2e
1 Fairbairn et al. AHA 2025.
2 Rinehart SJ, et al. DECIDE Primary Outcomes. J Cardiovasc Comput Tomogr. 2025; 19(4):S78-79. doi.org/10.1016/j.jcct.2025.05.185
3 Collins et al. Lancet 2016. DOI: 10.1016/S0140-6736(16)31357-5
4 Fairbairn et al. HEART. 2025. doi:10.1136/heartjnl-2025-BSCI.5

Heartflow Plaque Staging Personalizes Individual Cardiac Risk
*Heartflow Plaque Analysis is an FDA-cleared device. Heartflow Plaque Staging is an investigational only framework and its safety and effectiveness have not been reviewed by the FDA.
Overall Considerations
3. Tzimas, et al. JACC. 2023. https://doi.org/10.1016/j.jcmg.2023.05.011
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.